Volume XLVIII, Number 2
Authors
Andrea Pescina Liberale
University of Houston
Jamison V. Kovach
University of Houston
Introduction
Research activities, such as clinical trials for example, involve enrolling human subjects as volunteers to participate in research studies (Gabriele, 2000). Given that a substantial portion of research is sponsored by the federal government through money collected from U.S. taxpayers, investigators and research institutions have a responsibility to ensure research funding is not used in ways that harm or unnecessarily risk harming subjects participating in research studies. Given the volume of regulations, guidelines, etc. regarding research involving human subjects at the federal, state, and local levels, however, it can often be challenging to maintain compliance (Steinert, 2002). This research investigates how to reduce the time to review and render approval/ denial decisions for research protocols that involve human subjects.
To ensure the ethical conduct of research involving human subjects, institutions that receive federal funding for research are required to establish an institutional review board (IRB). The intent of the IRB is to protect the ethical rights and welfare of human subjects from research risks through the initial and continuing review of research protocols, adverse events, amendments, and other issues. The policies and procedures used to guide this process within each institution assists investigators with maintaining compliance with federal regulations (Steinert, 2002). That is, the modifications to research protocols, consent forms, recruitment materials, etc. often requestedby IRB committees are meant to balance the risks and benefits of a proposed research protocol (Gabriele, 2003; Gearhart, 2010).
Due to the competitiveness of research environments, it is imperative that institutions continuously improve their administrative support processes in order to help investigators effectively fulfill the requirements associated with their research activities (Kakande & Namirembe, 2012). One reason for investigator dissatisfaction is often the long wait times associated with obtaining IRB approval/denial decisions for research protocols (Whitney et al., 2008). Following an action research approach (Reason & Bradbury, 2008), researchers worked closely with IRB program administrators at one leading public research university to reduce the time to obtain IRB approval/denial decisions. Through a case study, this research demonstrates how this issue was addressed using the Lean Six Sigma methodology, which is a structured, problem-solving approach to improve process performance that combines Lean and Six Sigma tools to increase quality and reduce waste (Pepper & Spedding, 2010). Improving the efficiency of this process helps investigators conduct their research in a timelier manner, while also ensuring compliance.
The following sections provide details on the background, methodology, and the case study with results and conclusions. In the background section, information is provided regarding the IRB review process and some methods others have used previously to improve this process. The methodology section discusses the approach used to guide this research. This is followed by the case study section, which provides a detailed description of the work performed to improve the IRB review process. It also includes a description of the institution in which this research was conducted, as well as a discussion regarding how each phase of the Lean Six Sigma methodology was applied. Finally, the results of this improvement effort are summarized along with some concluding remarks regarding the implications of this research and areas for future work.
Background
Protocols for research involving human subjects typically fall into one of three review categories: 1) exempt (i.e., studies involving collecting new or existing data in such a manner that subjects cannot be identified, which are reviewed only by IRB program administrators); 2) expedited (i.e., studies posing minimal risks to human subjects, such as the collection of biological specimens via noninvasive means, which are reviewed by only one IRB committee member); or 3) full review (i.e., studies involving a larger range/higher level of potential risks to human subjects that do not qualify as exempt or expedited, which are reviewed by a full IRB committee) (Steinert, 2002). A recent study by the Association for the Accreditation of Human Research Protection Programs (AAHRPP, 2015) found that in the U.S. research protocols requiring full review take the longest amount of time (i.e., the median time from submission to an approval/denial decision is 39 days).
Many factors play a role in determining how long it takes to obtain IRB approval/denial decisions for a research protocol. There is a large body of regulatory requirements that must be referenced during research protocol reviews. Protocols must also be reviewed prior to the initiation of research activities and annually thereafter for on-going protocols (Gabriele, 2000). In addition, IRB program administrators and/or committee members often have heavy workloads. For example, nearly 40 percent of research intuitions have only one IRB committee, and a median of 471 protocols require full review by each institution annually in the U.S. (AAHRPP, 2015). Given this workload, there can often be insufficient time for IRB program administrators and/ or committee members to meticulously review protocols (Gabriele, 2000). Furthermore, IRB committee meetings often tend to be time consuming, and/or it can be difficult to obtain a quorum. Both of these issues contribute to long wait times for consideration of new protocols or reconsideration of revised protocols.
Investigators also contribute to the length of time needed for the IRB review process by not always providing sufficient information in their research protocols so they can be adequately reviewed. This situation, unfortunately, often leads to multiple cycles of revisions and re-reviews (Blustein, Regenstein, Siegel, & Billings, 2007; Green, Lowery, Kowalski, & Wyszewianski, 2006; Shalala, 2000). This situation is often further exacerbated when institutions’ IRB program administrators have insufficient resources and/or poorly organize/use available resources to support the review process (Andrews, Moore, Mean, & Weinberg, 2012).
Suggestions for reducing the time to obtain IRB approval/denial decisions include encouraging investigators to start working on their IRB applications as early as possible. Also, they can schedule meetings with IRB committee members to obtain guidance regarding potential risks and how to address them in advance of submitting their research protocol for review. Providing templates that address typical issues covered in IRB applications, including research questions, study design, sampling approach, recruitment procedures, and consent processes/forms, that investigators can tailor to meet their needs have been used previously to streamline the review process (Blustein et al., 2007). Finally, to avoid imposing requirements that are not appropriate for the proposed research, which unnecessarily extends the revision and re-review process, systems are needed to ensure IRB committee members have the correct experience and understanding to conduct reviews for their assigned protocols (Green et al., 2006).
To improve the IRB review process, one research institution recently developed metrics within a structure-process-outcome model to systematically analyze various aspects of their process for efficiency and determine the ethical issues that tend to prolong the review process (Adams et al., 2014). Another institution addressed this problem by reducing the size of each IRB committee, doubling their total number of committees, increasing the frequency of committee meetings, but scheduling meetings for shorter periods of time. This approach not only reduced the time to obtain IRB approval/denial decisions by nearly 50 percent, but it also increased the quality of reviews because more frequent meetings with shorter agendas allowed committee members to more carefully review each protocol (Andrews et al., 2012).
Research Context
This research was conducted at the University of Houston, which has approximately $100 million in research expenditures annually. In accordance with federal regulations, the University has an IRB review process that is managed by staff in the Division of Research’s Office of Research Policies, Compliance, and Committees. Faculty, staff, and students proposing to engage in any research activity involving the use of human subjects must obtain IRB approval prior to the recruitment for and initiation of research procedures. To handle the large volume of research protocols that require full review, the University of Houston has established three separate IRB committees. Each committee follows the same review process, and individual research protocols are reviewed by one of these three committees depending on the college from which the protocol was submitted. Each IRB committee has approximately 10 members with at least five alternate members. Members of these committees include representatives from the colleges and departments that submit to the committee, members not affiliated with the University of Houston, graduate students, a prisoner representative, and administrators that work in the Division of Research.
Unfortunately, the IRB review process for research protocols that require full review at the University of Houston has a median value of over 50 days. This is more than 10 percent longer than the median time in the U.S. to render an IRB approval/denial decision. In order to better serve those conducting research involving human subjects and increase their capacity to support research activities, the Division of Research undertook a Lean Six Sigma project to reduce the time to obtain IRB approval/denial decisions.
Methodology
This research applied the Lean Six Sigma methodology to reduce the time to obtain IRB approval/ denial decisions for a research protocol through a case study. The aim of Lean and Six Sigma are to reduce waste and variation within operational processes (Schroeder, Linderman, Liedtke, & Choo, 2008; Shah & Ward, 2007). Six Sigma alone was originally developed as a structured problem solving approach for use in production environments (Pande, Neumann, & Cavanagh, 2000). More recently, however, Lean and Six Sigma approaches have evolved into a combined methodology for improving process efficiency and reducing defects/errors/mistakes (Pepper & Spedding, 2010). The combined approach, Lean Six Sigma, has been used successfully to improve process performance in a variety of industries including services operations such as financial services (de Mast, Kemper, Wiltjer, & Does, 2013), construction (Anderson & Kovach, 2014), and education (Kulkarni & Kovach, 2016).
Process improvement projects using the Lean Six Sigma methodology follow a structured approach that has five phases: Define, Measure, Analyze, Improve, and Control (DMAIC). In the Define phase, the problem/opportunity for improvement and the key stakeholders are identified. At this point, a project team is also established, and management approval for the project is obtained. The Measure phase includes mapping the process, analyzing the measurement system, and establishing a baseline measurement regarding the current process performance. During the Analyze phase, the project team identifies the potential causes of the problem and evaluates them using process and/or statistical analysis methods to determine the vital few root cause(s). The Improve phase consists of identifying potential solution(s) to address the root cause(s) identified previously, narrowing them down to the sub-set of solutions that best addresses the problem at hand, and fully implementing the selected solution(s). Finally, the Control phase involves establishing a control plan to ensure that the changes implemented regarding how work is to be performed will be sustained and the performance gains made through the project will not be lost over time (de Mast & Lokkerbol, 2012; Hahn, Doganaksoy, & Hoerl, 2000).
Using this methodology, the University of Houston’s Division of Research engaged in a participatory action research method of inquiry that involved staff and researchers working together. The aim of action research is to develop practical solutions to a pressing problem through the integration of action and reflection (Reason & Bradbury, 2008). Using this type of approach provided an opportunity for researchers to function as participants in the University’s Lean Six Sigma project through meetings and office visits; hence, researchers, along with staff at the University, were involved in analyzing the IRB review process to identify cause(s) of waste and together implemented changes to reduce the time to obtain approval/denial decisions for a research protocol. Within this framework, the action research process of planning, taking action, and evaluating the action, which leads to planning for further action was used to ensure that what was learned from one phase of the project was then used as the input to the subsequent phase (Coughlan & Coghlan, 2002).
In the end, the Division of Research felt that the improvements made to streamlining their IRB review process would provide them with additional capacity to support research activities without having to hire additional staff; hence, the main question guiding this research was how does the University need to change their IRB process to effectively reduce the time to review a research protocol? The next section provides a detailed account the work done in each phase of the DMAIC methodology to develop a practical solution to the problem of long wait times for investigators to obtain IRB approval/denial decisions for their research protocols.
Case Study
Define Phase
The team involved in this project included staff working in the IRB review process, and it was led by the Assistant Director of the Office of Research Policies, Compliance, and Committees. Together, this team developed a project charter that identified the need for the project, as well as the responsibilities of the team members involved. To describe the situation at the beginning of the project and the project’s specific objectives, the following problem and mission statements were developed:
Problem statement: The University of Houston’s process to obtain IRB approval/denial decisions for research protocols that require a full committee review has averaged 52.27 days over the last six months ( July–December 2015), resulting in delays and/or cancellations of research activities/studies.
Mission statement: Reduce the average time to obtain IRB approval/denial decisions for research protocols that require a full committee review to 46 days or less (a 15 percent reduction) over the next nine months (by September 2016), resulting in fewer delays and/or cancellations of research activities/studies.
Measure Phase
In this phase of the project, the team outlined the IRB review process using a suppliers, inputs, process, outputs, and customers (SIPOC) diagram, as shown in Figure 1. The center column of Figure 1 describes the general steps required to approve a research protocol that requires a full IRB committee review. First, the Division of Research’s Office of Research Policies, Compliance, and Committees receives a protocol, and a pre-review is conducted to confirm:
- The review category specified is correct,
- All required documents were submitted, and
- The required training has been completed by the investigator.
Then, the research protocol is assigned to an IRB committee, and the primary and secondary reviewers (i.e., IRB committee members) are assigned. Once the reviews are complete, the IRB committee meets to analyze the protocol and its review, and they render a decision. Then, the decision is sent to the investigator. If modifications were requested, the investigator revises and resubmits the research protocol, which is reviewed again. Finally, the revised protocol and its review are analyzed in an additional IRB committee meeting, and if no further modifications are needed, the committee renders a final decision. If approved, the investigator can then begin their research activities following the approved protocol.
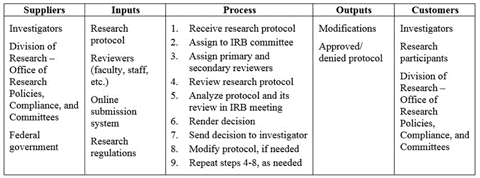
Figure 1. High-level view of the IRB review process
As additionally shown in Figure 1, the inputs for the IRB review process are the research protocol, reviewers (i.e., faculty, staff, etc.), online submission system, and research regulations, which are supplied by researchers, the Division of Research’s Office of Research Policies, Compliance, and Committees, and the federal government. The outputs of this process are protocol modifications and the approved/denied protocol. These outputs are used by researchers, research participants, and the Division of Research’s Office of Research Policies, Compliance, and Committees. To ensure the project team had a detailed understanding regarding how the process operates, a process map illustrating the step-by-step nature of the IRB review process was also created, which was three pages long (not included due to space limitations).
To measure the current performance of the IRB process regarding the time to obtain an approval/ denial decision for a research protocol that requires a full committee review, date stamp data for protocols processed July through December 2015 were collected from the University’s research administration database. During this time, nearly 70 research protocols that required a full IRB committee review were processed. The process cycle time was calculated by subtracting the date a research protocol was submitted from the date the protocol approval/denial decision was sent to the investigator. The time to obtain IRB approval/denial decisions for protocols that required a full committee review varied from 11 days to 127 days with an average of 52.27 days and a standard deviation of 33.23 days. Instances where it took approximately 100 days or more to obtain IRB approval/denial decisions occurred for approximately 15 percent of the research protocols reviewed.
Analyze Phase
To identify the root cause(s) of excessive time to obtain IRB approval/denial decisions for a research protocol that requires a full committee review, the project team utilized failure modes and effects analysis (FMEA) to analyze the process. FMEA is an analytical tool that identifies and prioritizes potential failures in a process (Stamatis, 2003). Using this tool, the team determined how each step in the process could fail, the effect(s) and cause(s) of the failure, and the current control(s) in the process that may help detect/prevent the failure. The project team also utilized a 10-point rating scale to evaluate each failure according to:
- The severity of the effect, where “10” represents a catastrophic event and “1” represents an issue that is not noticeable to the customer;
- The occurrence of the potential cause, where “10” represents the cause is almost certain to occur and “1” represents it is highly unlikely the cause will occur; and
- The current control’s ability to detect/prevent the failure, where “10” represents the control is nearly certain not to detect the failure/no controls and “1” represents the control is almost certain to detect the failure.
Once all aspects of the IRB review process had been evaluated, the failures identified were prioritized based on their risk priority number (RPN), which is calculated by multiplying the ratings for severity, occurrence, and detection, in order to determine which items in the process represent the highest risk of failure. The items with the highest RPNs are shown in Table 1. These items represent the failures with highest risk of causing delays within the IRB review process. The causes associated with these failures include:
- Missing coversheet and
- Not enough time for Coordinators, i.e., IRB process administrators, to conduct the protocol pre-review.
Based on the RPN values, the project team identified these two items as the root causes for excessive time to obtain IRB approval/denial decisions.

Table 1. Top-rated Failures Associated with IRB Review Process Delays
Improve Phase
After determining the root causes, the project team began to compile a list of potential solutions to address each of these issues. When considering root cause 1 (missing coversheet), one idea the project team identified was to eliminate the need for a coversheet explaining how the research protocol had been revised. As the team investigated how this could be accomplished, they learned that the University was in the process of implementing a new online system for managing the IRB review process. When the project team inquired about the operations of this new system, they learned that it had the capability to show the original research protocol compared with the revised version in a split screen. This optional function in this new system would make it easy for Coordinators to understand how the protocol was revised to address requested modifications during their pre-review. To eliminate the need for a coversheet to be submitted with revised research protocols, the project team recommended this function be enabled in the new online system.
For root cause 2 (not enough time for Coordinators to conduct the protocol pre-review), the project team held a brainstorming session. Each team member individually prepared a list of potential solution ideas. During the brainstorming session these ideas were shared and other ideas were developed by the team. In the end, the project team identified six ideas to address the need for creating more time for Coordinators to conduct protocol pre-reviews. Nominal group technique was then used to prioritize this list of solution ideas. That is, once project team members had individually rank-ordered the ideas developed, this information was compiled, the sum of individual rankings for each solution idea were calculated, and the ideas were prioritized based on their total score from highest to lowest, as shown in Table 2. The solution with the highest total score was “delegate Coordinator’s other responsibilities to student workers” (i.e., lower-skilled tasks) so Coordinators would have more time to conduct protocol pre-reviews (i.e., a high-skilled task).
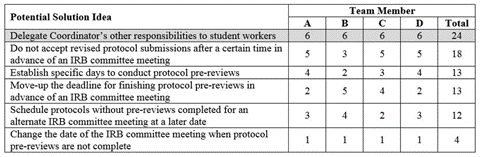
Table 2. Prioritized Potential Solutions for Root Cause 2 – Not Enough Time to Conduct the Protocol Pre-review
In order to delegate some of the Coordinator’s time consuming, lower-skilled tasks to others involved in the IRB process, a brainstorming session was held to identify which activities could be completed by student workers. The tasks identified included:
- Using checklists to ensure required documents and training have been submitted/completed for each research protocol;
- Sending letters, i.e., protocol approvals, closures, and confirmations for non-human subjects research;
- Filing approval memos; and
- Managing the IRB process’ common e-mail inbox.
In order to ensure everyone involved would understand the new way of performing this work, a description for each of these new tasks was developed and the people responsible for each of these tasks were identified. Over the next week, Coordinators trained student workers on how to perform these activities.
Control Phase
In order to sustain the improvements made to the IRB process as a result of this project, a control plan was established that included documenting, training, auditing, and monitoring mechanisms for the improved process. As previously discussed, the work activities delegated to student workers were documented and the student workers were trained by the Coordinators regarding how to perform these tasks. An overview of the audit and monitoring aspects of the control plan are shown in Table 3. The items listed provide a structure for process monitoring that specifies the steps, or work, in the IRB review process to be performed along with the control methods, their frequency, and the owners, i.e., those responsible for overseeing each control mechanism. Similarly, the last few columns of Table 3 specify the methods to audit the process control mechanisms, their frequency, and the assigned owner, as well as the person to which the audit results are to be reported.
As shown in Table 3, for example, the process step “conduct protocol pre-review” will be controlled by checking that all pre-reviews are complete a week before the IRB committee meeting, and this will be done bi-weekly by the Coordinators. In addition, this control activity will be audited by reviewing the protocol pre-review report, which will be done bi-weekly by the Assistant Director of the Office of Research Policies, Compliance, and Committees, and the audit results will be reported to the Director. Finally, it was determined that a run chart, similar to that depicted in Figure 2 (shown in the next section), would be used to monitor the performance of the improved process relative to the time to obtain an IRB approval/denial decisions for a research protocol.

Table 3. Control Plan for Auditing and Monitoring the Improved IRB Review Process
Results
After the new online system had been implemented and students working in the IRB process had been completing the new tasks delegated to them on their own for approximately three months, data were collected from the University’s research administration database to determine the performance of the improved IRB review process. Figure 2 depicts a run chart of the time to obtain IRB approval/denial decisions, i.e., the date a protocol approval/denial decision was sent to the investigator minus the date the research protocol was submitted, for July 2015–September 2016. During this time, more than 120 research protocols that required a full IRB committee review were processed. The data shown in Figure 2 from July–December 2015 represents the baseline measurement for this project, which was discussed previously in the Measure phase. The data shown from January–May 2016 depicts the performance of the IRB review process while the project team conducted their investigation of the process to identify the root causes and develop solution ideas. Finally, the data shown from June–September 2016 represents the performance of the improved IRB review process after the solutions identified through this project were implemented. As a result of this project, the time to obtain IRB approval was reduced from an average of 52.27 days with a standard deviation of 33.23 days at the beginning of the project ( July– December 2015) to an average of 46.96 days with a standard deviation of 20 days by the end of the project ( June–September 2016). These data provide initial evidence that the improvements implemented through this project were effective in terms of reducing the time to obtain an IRB approval/denial decision.
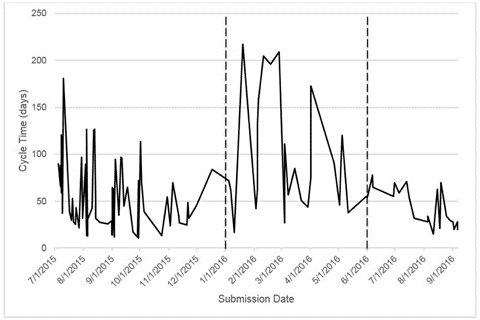
Figure 2. Performance of the IRB review process over time.
Conclusion
Through a case study at one institution, this research demonstrated how to apply the Lean Six Sigma methodology to improve the IRB review process. This study successfully identified what the university in which this research was conducted needed to do to reduce the time to obtain IRB approval/denial decisions for research protocols that require full review. Through this research, the time to obtain IRB approval/denial decisions was reduced by approximately 15 percent. This was achieved through two actions. First, the project team recommended enabling a feature within the new online system for managing the IRB process to make it easier for Coordinators to check the revisions made to research protocols in response to requested modifications during their pre- review. Without this specific recommendation, it is not clear whether the other group responsible for implementing the new system would have recognized the importance of and enabled this particular system feature on their own. Second, the project team helped Coordinators delegate some of their time consuming, lower-skilled tasks to student workers to create more time for Coordinators to conduct protocol pre-reviews.
Because this research describes a case study performed in just one research institution, the results obtained may not be generalizable to all IRB review processes. However, the approach may be useful to other IRB program administrators and/or committee members to provide guidance for conducting similar process improvement efforts within their institutions in the future. Finally, given that the case study conducted in this research focused on research protocols that require full IRB committee review, the next step for the university that participated in this research is to use the principles and practices of Lean Six Sigma to further improve their IRB review processes for research protocols that require exempt and/or expedited reviews.
Authors’ Note
The research described in this article is original work that has not been derived from another source. The authors wish to thank the following representatives from the University of Houston’s Office of Research Policies, Compliance, and Committees who supported this project from beginning to end: Ms. Jennifer Edge and all those working in the IRB approval process. Without their support, this project would not have been possible.
Andrea Pescina Liberale
Master’s Candidate
University of Houston
4730 Calhoun Rd. Room 300
Houston, Texas 77204, U.S.A.
Phone: 713-743-1704
Fax: 713-743-4032
Email: aapescinaliberale@uh.edu
Jamison V. Kovach
Associate Professor
University of Houston
4730 Calhoun Rd. Room 300
Houston, Texas 77204, U.S.A.
Phone: 713-743-1704
Fax: 713-743-4032
Email: jvkovach@uh.edu
Correspondence regarding this article should be addressed to Jamison V. Kovach, Associate Professor, University of Houston, 4730 Calhoun Rd. Room 300, Houston, Texas 77204, U.S.A., jvkovach@uh.edu
References
AAHRPP. (2015). Metrics on human research protection program performance. Washington, D.C.: Association for the Accreditation of Human Research Protection Programs.
Adams, P., Kaewkungwal, J., Limphattharacharoen, C., Prakobtham, S., Pengsaa, K., & Khusmith, S. (2014). Is your ethics committee efficient? Using “IRB metrics” as a self- assessment tool for continuous improvement at the Faculty of Tropical Medicine, Mahidol University, Thailand. PloS one, 9(11), e113356. https://doi.org/10.1371/journal.pone.0113356
Anderson, N. C., & Kovach, J. V. (2014). Reducing welding defects in turnaround projects: A Lean Six Sigma case study. Quality Engineering, 26(2), 168-181. http://dx.doi.org/10.1080/08982112.2013.801492
Andrews, J. E., Moore, J. B., Mean, P., & Weinberg, R. B. (2012). An IRB transformation: Increasing quality and efficiency using existing resources. Journal of Research Administration, 43(2), 69-81.
Blustein, J., Regenstein, M., Siegel, B., & Billings, J. (2007). Notes from the field: Jumpstarting the IRB approval process in multicenter studies. Health Services Research, 42(4), 1773- 1782. doi:10.1111/j.1475-6773.2006.00687.x
Coughlan, P., & Coghlan, D. (2002). Action research for operations management. International Journal of Operations and Production Management, 22(2), 220-240. https://doi.org/10.1108/01443570210417515
de Mast, J., Kemper, B. P., Wiltjer, A., & Does, R. J. (2013). Quality quandaries: Deploying operational excellence at a financial service provider. Quality Engineering, 25(3), 298-306. http://dx.doi.org/10.1080/08982112.2013.783599
de Mast, J., & Lokkerbol, J. (2012). An analysis of the Six Sigma DMAIC method from the perspective of problem solving. International Journal of Production Economics, 139(2), 604-614. https://doi.org/10.1016/j.ijpe.2012.05.035
Gabriele, E. F. (2000). Tending the ground of our being: The IRB and IRB administration in the biomedical research culture. Journal of Research Administration, 1(1), 17-21.
Gabriele, E. F. (2003). The Belmont ethos: The meaning of the Belmont principles for human subject protections. Journal of Research Administration, 34(2), 19-24.
Gearhart, C. (2010). The role of IRBs in research. Applied Clinical Trials, 9(7), 4-5.
Green, L. A., Lowery, J. C., Kowalski, C. P., & Wyszewianski, L. (2006). Impact of institutional review board practice variation on observational health services research. Health Services Research, 41(1), 214-230. doi:10.1111/j.1475-6773.2005.00458.x
Hahn, G. J., Doganaksoy, N., & Hoerl, R. (2000). The evolution of Six Sigma. Quality Engineering, 12(3), 317-326. http://dx.doi.org/10.1080/08982110008962595
Kakande, N., & Namirembe, R. (2012). Strengthening institutional research administration in Uganda: A case study on developing collaborations among academic and research institutions. Journal of Research Administration, 43(1), 39-59.
Kulkarni, A., & Kovach, J. V. (2016). Improving room scheduling for special events in academic environments–A Lean Six Sigma case study. Quality Approaches in Education, 7(2), 22-29.
Pande, P. S., Neumann, R. P., & Cavanagh, R. R. (2000). The Six Sigma way: How GE, Motorola, and other top companies are honing their performance. New York: McGraw- Hill.
Pepper, M. P. J., & Spedding, T. A. (2010). The evolution of Lean Six Sigma. International Journal of Quality and Reliability Management, 27(2), 138-155. https://doi.org/10.1108/02656711011014276
Reason, P., & Bradbury, H. (2008). The Sage handbook of action research: Participative inquiry and practice (2nd ed.). London: Sage Publications.
Schroeder, R. G., Linderman, K., Liedtke, C., & Choo, A. S. (2008). Six Sigma: Definition and underlying theory. Journal of Operations Management, 26(4), 536-554. https://doi.org/10.1016/j.jom.2007.06.007
Shah, R., & Ward, P. T. (2007). Defining and developing measures of Lean production. Journal of Operations Management, 25(4), 785-805. https://doi.org/10.1016/j.jom.2007.01.019
Shalala, D. (2000). Protecting research subjects - what must be done. New England Journal of Medicine, 343(11), 808-810. Retrieved from http://www.columbia.edu/itc/history/rothman/COL479D0003.pdf
Stamatis, D. H. (2003). Failure Mode and Effect Analysis: FMEA from theory to execution (2nd ed.). Milwaukee, WI: Quality Press.
Steinert, B. W. (2002). Protection of human subjects: A primer for the new administrator. Journal of Research Administration, 33(2), 67-74.
Whitney, S. N., Alcser, K., Schneider, C. E., McCullough, L. B., McGuire, A. L., & Volk, R.
J. (2008). Principal investigator views of the IRB system. International Journal of Medical Sciences, 5(2), 68-72. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2288790/pdf/ijmsv05p0068.pdf
Keywords
Research administration, Institutional review board (IRB) approval/denial process, Lean Six Sigma, process improvement efforts
#VolumeXLVIIINumber2#ResearchAdministration#InstitutionalReviewBoard#approval/denialProcess#lean6Sigma#processImprovementEfforts